Applications MARS spectral CT Scanner
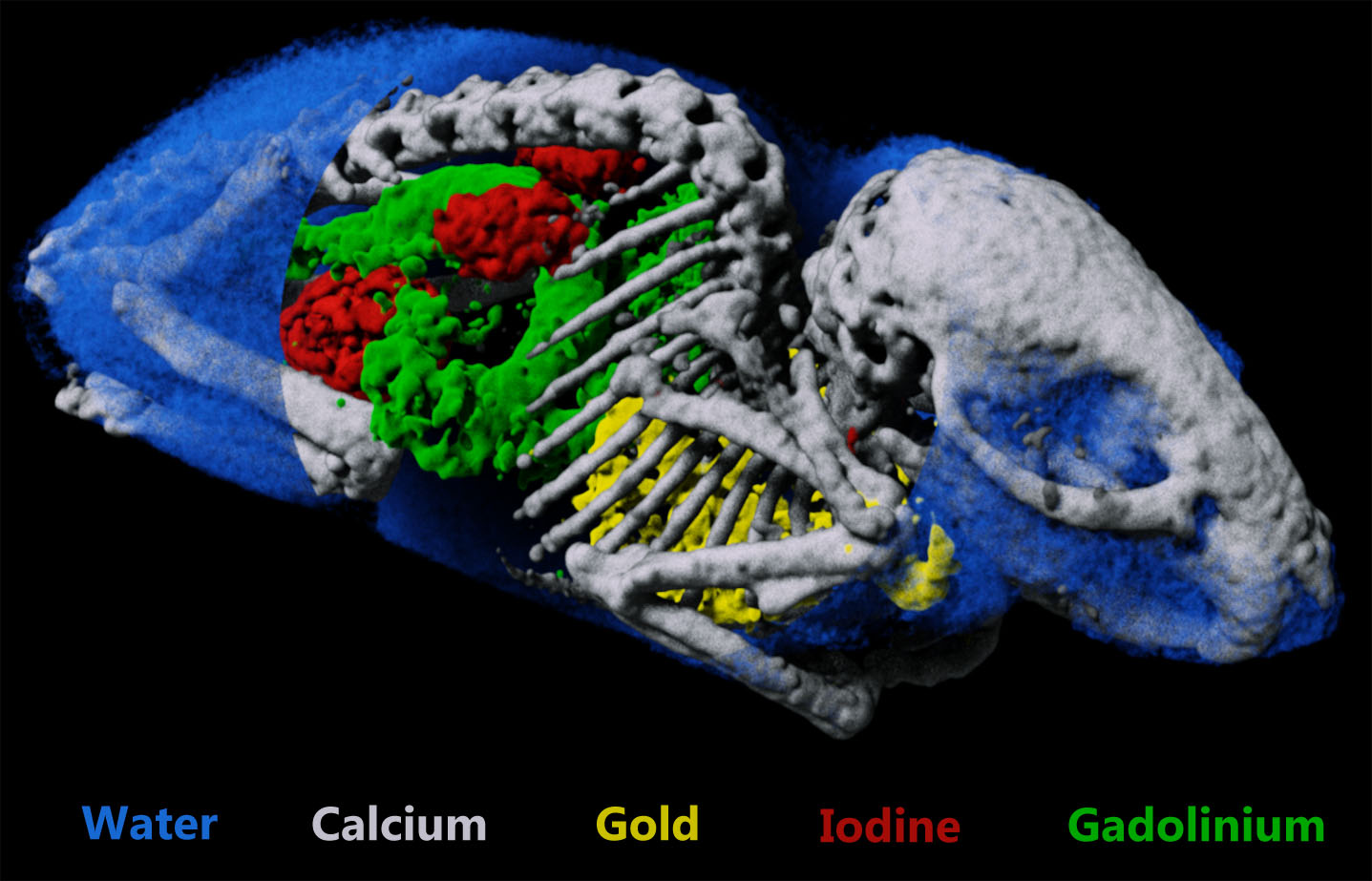
Measure multiple contrast agents simultaneously
CT has traditionally been limited to the use of a single contrast agent per scan. Spectral CT gives researchers a tool that can quantify a number of contrast agents as well as intrinsic markers such as lipid, bone and soft tissue. Tracking multiple biomarkers simultaneously provides a way to monitor multiple processes non-invasively.
Available data sets include mouse data and phantom data.
- R. Panta, et al (2018). Element-specific spectral imaging of multiple contrast agents: a phantom study. Journal of Instrumentation. 13 T02001. Read paper.
- M. Moghiseh, et al. (2016). Discrimination of Multiple High-Z Materials by Multi-Energy Spectral CT– A Phantom Study. JSM Biomed Imaging Data Pap 3(1): 1007. Read paper.
- N. Anderson, et al. Spectroscopic (multi-energy) ct distinguishes iodine and barium contrast material in MICE. European Radiology, vol. 20, pp. 2126–2134, 2010. Read paper.
- R .K. Roeder, et al. Probes for Molecular Imaging with Computed Tomography and Application to Cancer Imaging. Proc. SPIE, 10132, 101320X (2017). Read paper.
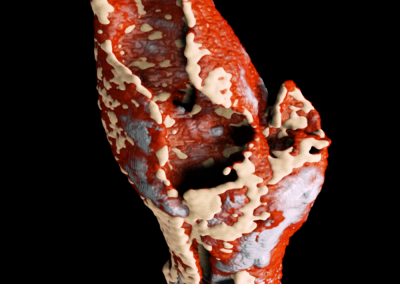
A new way to image cancer
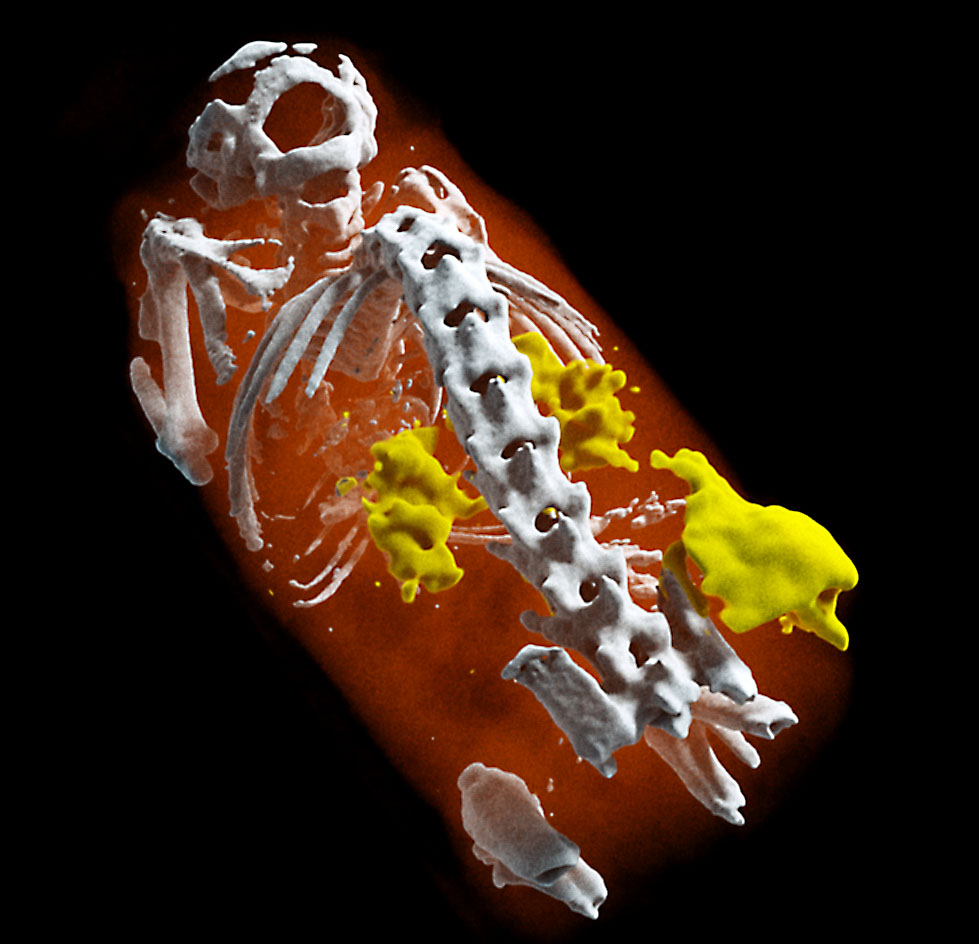
Lighting up tumour neovascularization using nanoparticles
A tumour was placed under the flank of the animal. Small gold nanoparticles (5 nm) were injected. The non-functionalized nanoparticles accumulate in areas of neovascularization (e.g. tumours) where vessels are more leaky.
MARS scanning opens the door to targeted imaging probes on CT.
Knowing whether an antibody-based treatment has reached its target tissue can be difficult. MARS spectral CT offers a method to track nanoparticles, allowing preclinical researchers to have confidence that their treatment has reached their target cells.
- Better characterisation of tumors
- Better monitoring of drug delivery
- Develop targeted imaging probes
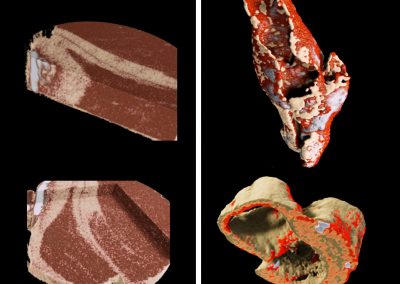
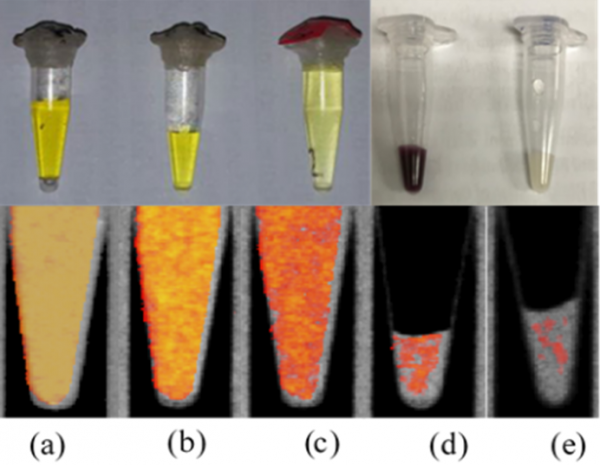
Imaging specific binding of antibody-gold nanoparticle complexes in vitro
(a), (b), (c) calibration standards of gold chloride
(d) Ovarian cancer cells incubated with gold nano-particles targeted to a surface marker of ovarian cancer (Rituximab)
(e) Ovarian cancer cells with gold nano-particles targeted to breast cancer (Herceptin)
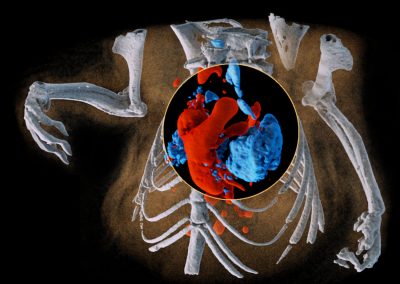
Better soft tissue discrimination
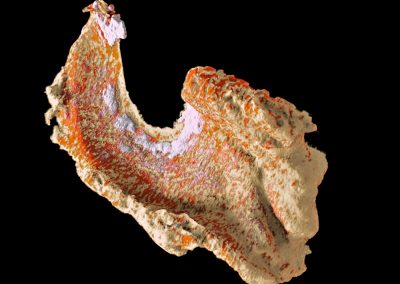
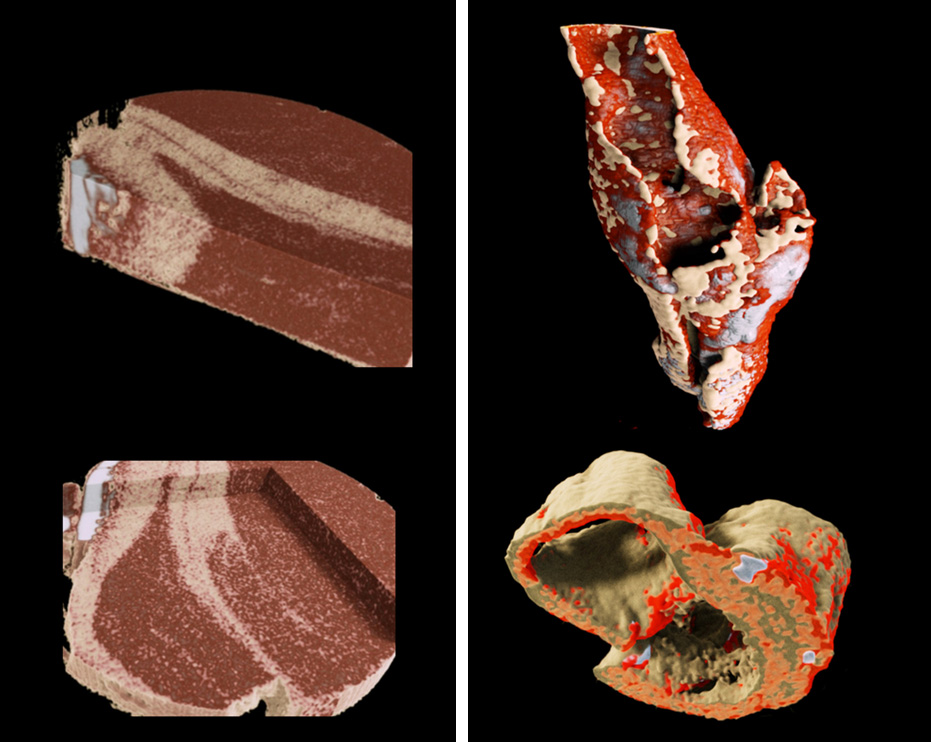
Spectral imaging provides better soft tissue contrast than is available with traditional x-ray systems. This enables imaging and distinguishing pathological features of cardiovascular disease at high spatial resolution, for example the components of atherosclerotic plaque. Alternatively it can be used to better characterise muscles, bone and fat.
Downloadable data set of lamb meat.
- R. Aamir, et al. MARS spectral molecular imaging of lamb tissue: data collection and image analysis. Journal of Instrumentation, vol. 9, no. 02, p. P02005. Read paper.
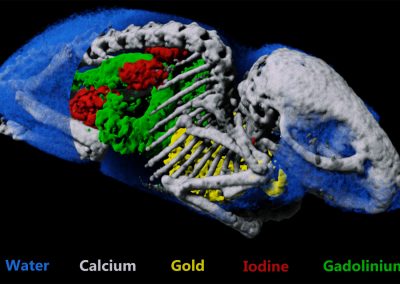
Structural and material information in a single scan
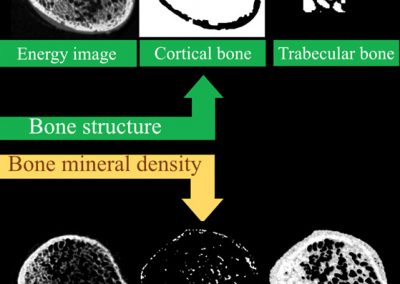
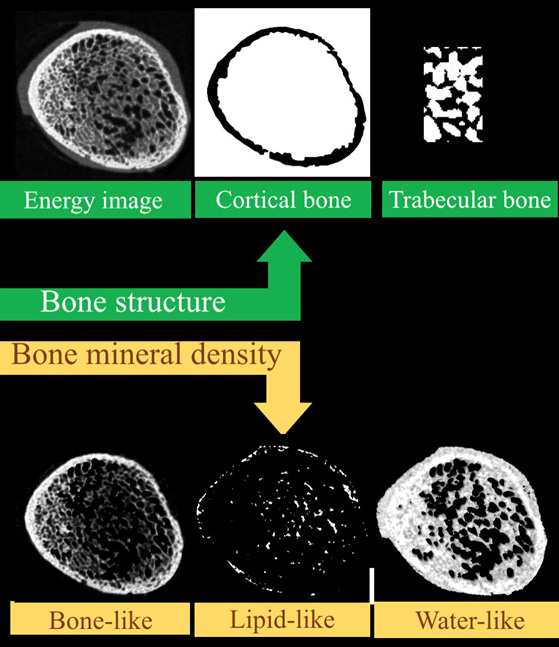
Why measure just structure or bone mineral density when you can do both?
Simultaneous measurement of both bone structure and bone composition using energy information and calcium, lipid and water channels.
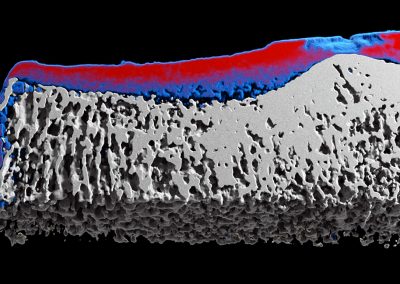
MARS enables both structural, and material information to be measured simultaneously. This means that bone mineralisation or bone densitometry can be measured within bone sites as well as architectural features such as cortical thickness, trabecular thickness, and trabecular spacing. Furthermore, some biomarkers of cartilage health can be measured including early measures of osteoarthritis.
Downloadable data sets include metallic scaffolds.
- M. Ramyar, et al. Establishing a method to measure bone structure using spectral CT. SPIE Medical Imaging, 2017. DOI: 10.1117/12.2255616. Read paper.
- M. Ramyar, et al. Establishing a method to measure bone density using spectral CT. Published by the European Congress of Radiology, 2017.
- K. Rajendran et.al. Reducing beam hardening and metal artefacts in spectral CT using Medipix3RX. Journal of Instrumentation, Vol. 9 P03015, March 2014. Read paper.
Molecular imaging without radiotracers
MARS promises to revolutionize diagnostic imaging by quantifying the elements and compounds of a sample in a single scan. It is the first commercially available 3D spectral (multi-energy) scanner to produce in vivo images with anatomic and molecular quantification at a fraction of the cost, time, and radiation dose of traditional molecular imaging, such as PET or SPECT.
The Medipix spectral x-ray detector technology was originally developed at CERN for the LHC and modified for medical applications; it is the result of 20+ years of collaborative innovation.
Examples of first Human Images
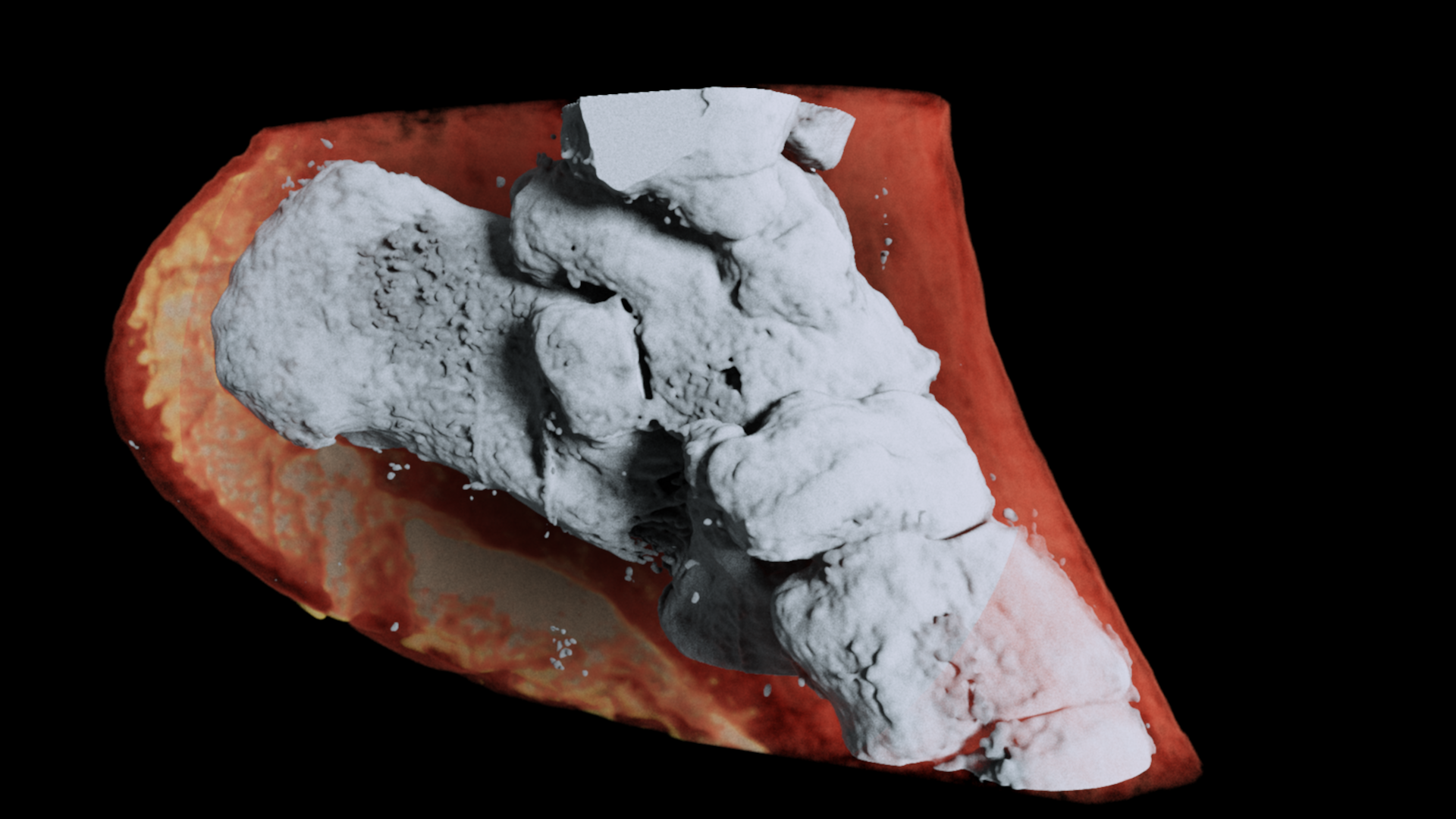
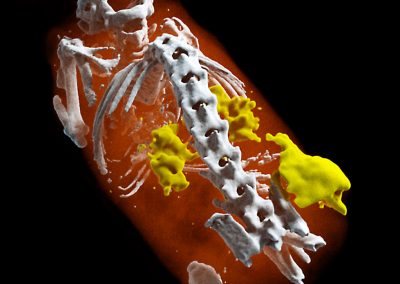
A 3D MARS image of an ankle viewed from the side where the soft tissue (coloured in red) has been made translucent to show the bones (white) and lipid-like material (yellow) inside the ankle.
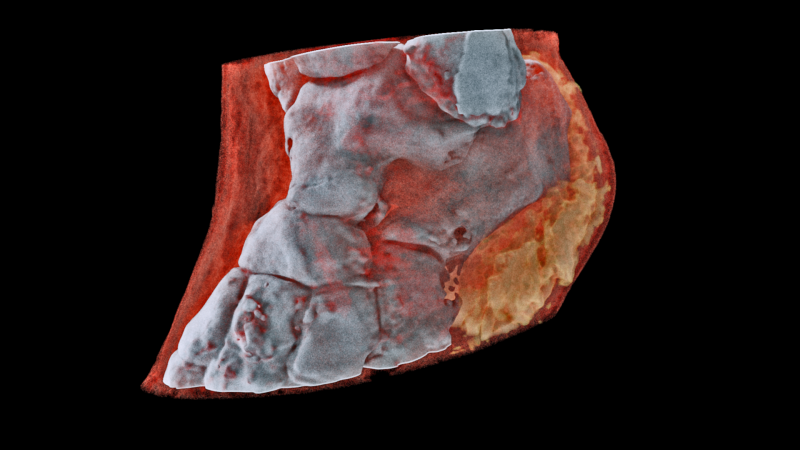
A 3D MARS image of an ankle viewed front on where the soft tissue (coloured in red) has been made translucent to show the bones (white) and lipid-like material (yellow) inside the ankle.
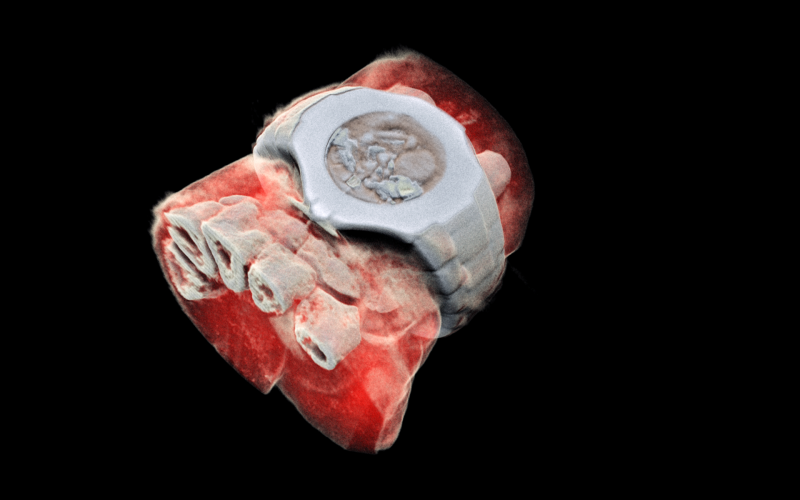
A 3D MARS image of a wrist with a watch showing part of the finger bones in white and soft tissue in red.
Building a solid foundation: MARS imaging for complex bone implant surgery
Advances in surgical technologies, such as robotics, implants, and targeted therapies, have facilitated the changing role of medical imaging. Imaging is no longer primarily used to diagnose a broken bone or to find out whether that swallowed toothpick made its way out alright. Imaging has moved towards therapy and intervention, including preoperative planning, post-operative assessment, designing custom bone implants, and image-guided surgery.
As most surgical technology now relies on some form of imaging, therein lies the opportunity.
This article will discuss the potential application of MARS spectral photon-counting computed tomography (MARS CT) in future bone implant surgeries.
If your foundation isn’t solid, it doesn’t matter how strong your house is – Paul Morrison, OSSIS Limited.
Joint replacement surgery has existed since the 1950s. Although we haven’t achieved “The Six Million Dollar Man” results just yet, the procedure can significantly improve the quality of life for patients with painful bone and joint diseases.
OSSIS Ltd is a New Zealand-based company that designs and manufactures patient-specific bone implants for very complex conditions, such as cancer. Preoperative planning for an oncological custom bone implant involves radiographers, radiologists, surgeons, and engineers. The team uses information from imaging (plain x-ray CT for bone and MRI for soft tissue) to find the margin between tumour and bone, design the patient-specific implant, and place the implant for long-term success.
Huge value lies in understanding bone health and surrounding tissues early – or building that solid foundation. This is why companies like OSSIS get excited about the development of MARS imaging.
MARS imaging could build the foundations necessary to create even better custom implants, its development is very exciting.
Advancements in medical imaging would enhance the surgical planning and patient-specific implant design resulting in a more predictable outcome for the patient.
Plain x-ray and CT scans measure density, which can show areas of scar tissue or hard bone but are vague in identifying important structures such as blood vessels and nerves. Moreover, the margin applied around a tumour is critical for removing all cancer, while at the same time saving every millimetre of healthy bone possible to increase the patient’s chance of a good long-term result. Tumour margins rely on information obtained by MRI, which suffers from poor spatial resolution.
MARS CT imaging could enable a deeper understanding of where ‘good’ bone is and where ‘bad’ bone is by quantitatively measuring calcium and water content, and other bone quality indicators. Studies have shown that knowing bone condition prior to surgery significantly influences the accuracy of implant placement (1).
The resolution of MARS imaging is much higher that MRI, about 35x higher in fact. Greater spatial resolution could increase the accuracy of tumour margins and improve patient outcomes. Furthermore, MARS imaging has the potential to assess osseointegration and implant revision by visualising the bone-metal interface due to reduced metal artefacts (image distortion).
The MARS team are incredibly excited to continue to explore the benefits of MARS imaging in orthopaedics/oncology, as improving healthcare is our goal. Check out the image gallery below of the different imaging techniques mentioned in this blog article!
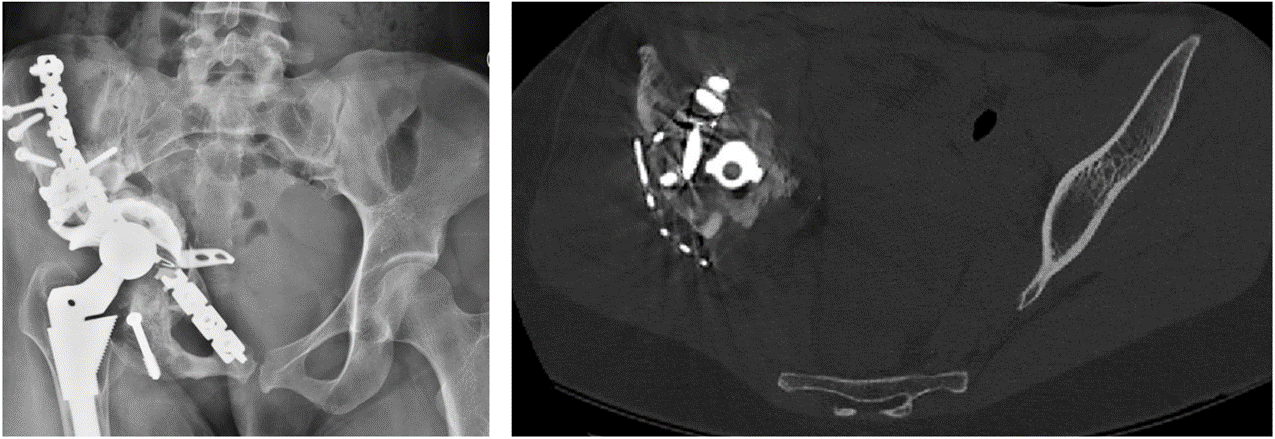
Left: Two-dimensional (2D) plain x-ray radiograph image. Plain x-ray provides high spatial resolution, with all anatomical structures overlaid.
Right: A single 2D image from a computed tomography (CT) scan of the same patient. The metal implant causes artefacts (image distortion) in the CT image due to beam hardening.
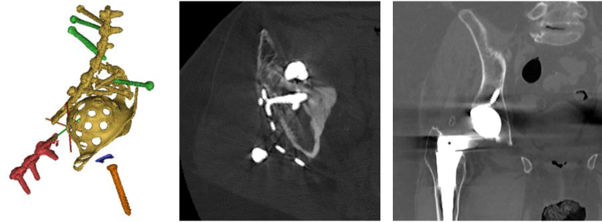
Left: CAD of a complex bone implant.
Centre: A single 2D image from a CT scan of the complex bone implant shown to the left.
Right: A single 2D image from a CT scan of a simple hip replacement
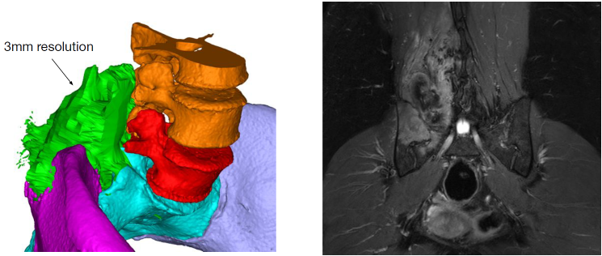
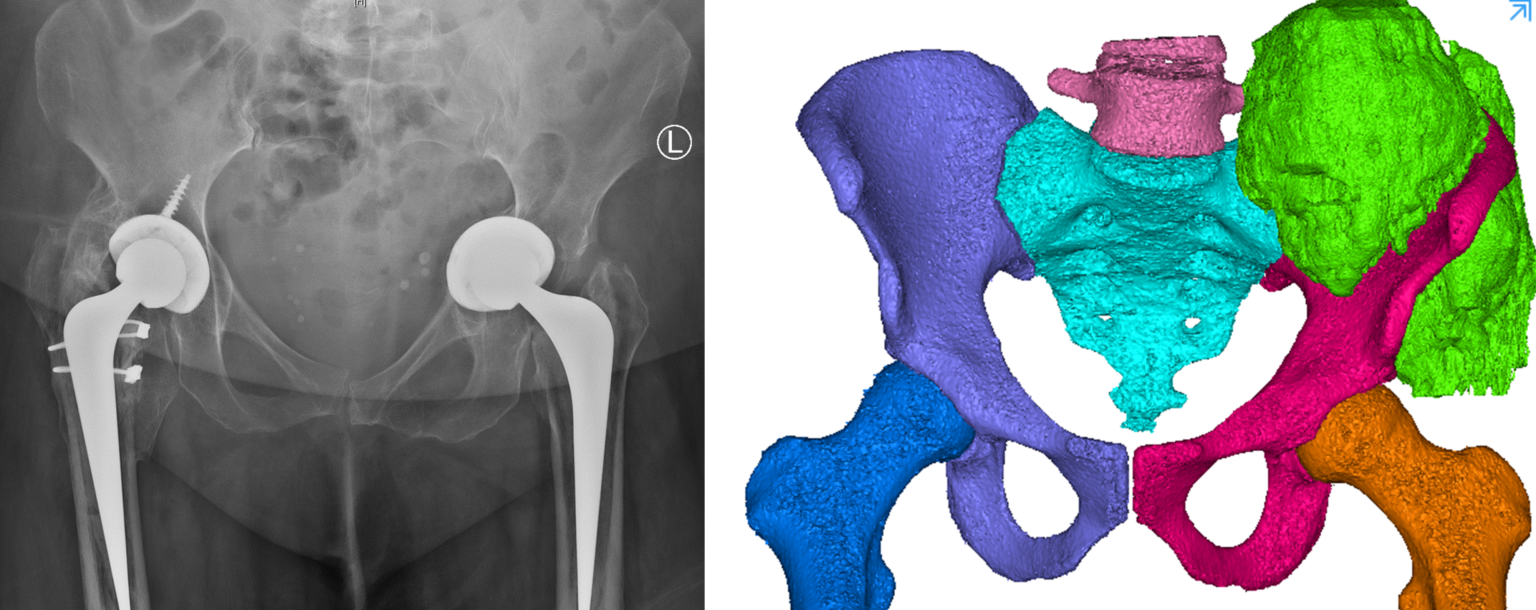
Left: 3D computer rendering of a CT scan and MRI combined. CT provides excellent information about bone, while MRI provides soft tissue (tumour in green) information. MRI has low spatial resolution (3 mm) compared to CT (300 microns). Oncological margins currently rely on MRI.
Right: A single 2D image from a MRI scan.
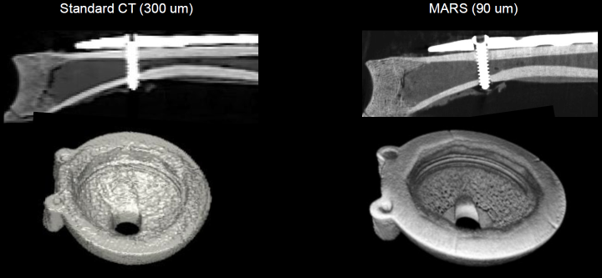
Left: Current CT imaging of a sheep clavicle with a titanium plate and screw, and metallic cup implant.
Right: The same objects scanned using MARS photon-counting CT – results show higher spatial resolution and reduced metal artefacts for improved bone-metal interface evaluation.
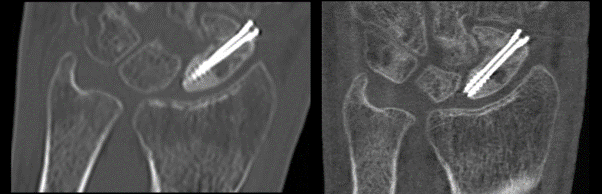
Left: A single 2D image from a current CT scan showing a fractured scaphoid bone with screw.
Right: A single 2D image from a MARS CT scan of the same wrist.
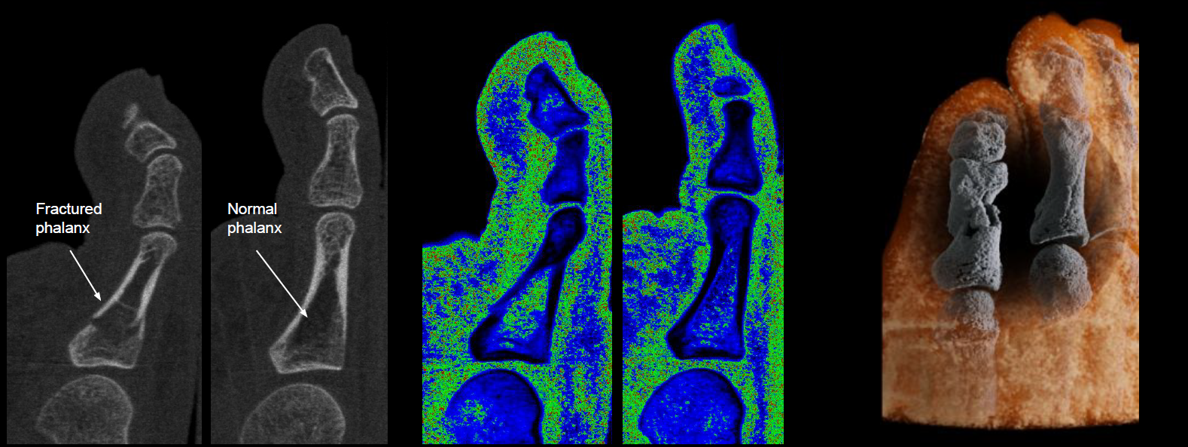
Left: 2D energy image from a MARS CT scan.
Centre: Energy information is converted to material information. 2D water channel image shows higher water content in the fractured region.
Right: 3D image showing calcium and fat channels. The MARS magic lens tool removes the fat channel to reveal the acute toe fracture within the calcium channel.